What is the difference between soap and detergent?
What are Detergents?
Soap is a compound made by combining Sodium Hydroxide or Potassium Hydroxide with vegetable oil or animal fats in a saponification reaction. Soaps are potassium or sodium salts of fatty acids with a long carbon chain. Soaps are naturally water-soluble.
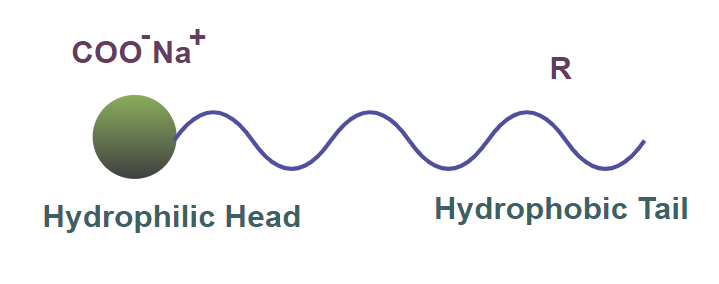
A soap molecule is made up of two components. These two components are referred to as:
- Hydrophobic tail: This part of the soap is water-repellent in nature and dissolves in oils. It is ionic in nature.
- Hydrophilic head: This part of the soap molecule is water attractive or water-loving and dissolves in water. It is made up of a long chain of hydrocarbons.
What are Soaps?
Detergents are compounds that have ionic groups connected to the end of a lengthy hydrocarbon chain. Detergents are long-chain carboxylic acid quaternary ammonium or sulfonate salts.
Detergents, often known as surfactants, are substances that lower water’s surface tension. Detergents were developed during World War 2 due to the lack of vegetable oils to make soaps. Detergents also contain two parts.
These are:
- Hydrophobic Tail: This part of the detergent is water-repellent similar to the soaps. It is the ionic or polar or charged group that is present at the end of the hydrocarbon chain.
- Hydrophilic Head: This part is water attractive or water-loving. It is made up of a long alkyl hydrocarbon chain.
Types of Detergents
Detergents are further classified into 3 types depending on the polarity of the polar group or hydrocarbon chain. These are:
- Cationic Detergents
- Anionic Detergents
- Non-ionic detergents
What is the difference between soap and detergent?
Soap is made from natural oils and fats and detergents normally are made from synthetic surfactants although there is an increasing number of detergents from natural derived ingredients
The fats and oils used in soapmaking come from animal or plant sources. Each fat or oil is made up of a distinctive mixture of several different triglycerides.

A detergent is an effective cleaning product because it contains one or more surfactants. Because of their chemical makeup, the surfactants used in detergents can be engineered to perform well under a variety of conditions. Such surfactants are less sensitive than soap to the hardness minerals in water and most will not form a film.
Detergent surfactants were developed in response to a shortage of animal and vegetable fats and oils during World War I and World War II. In addition, a substance that was resistant to hard water was needed to make cleaning more effective. At that time, petroleum was found to be a plentiful source for the manufacture of these surfactants. Today, detergent surfactants are made from a variety of petrochemicals (derived from petroleum) and/or oleochemicals (derived from fats and oils).